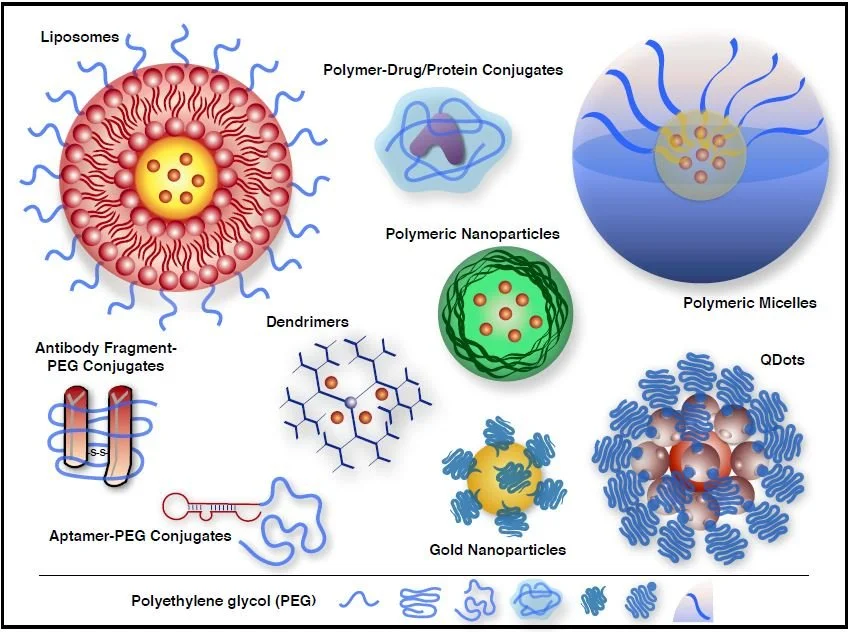
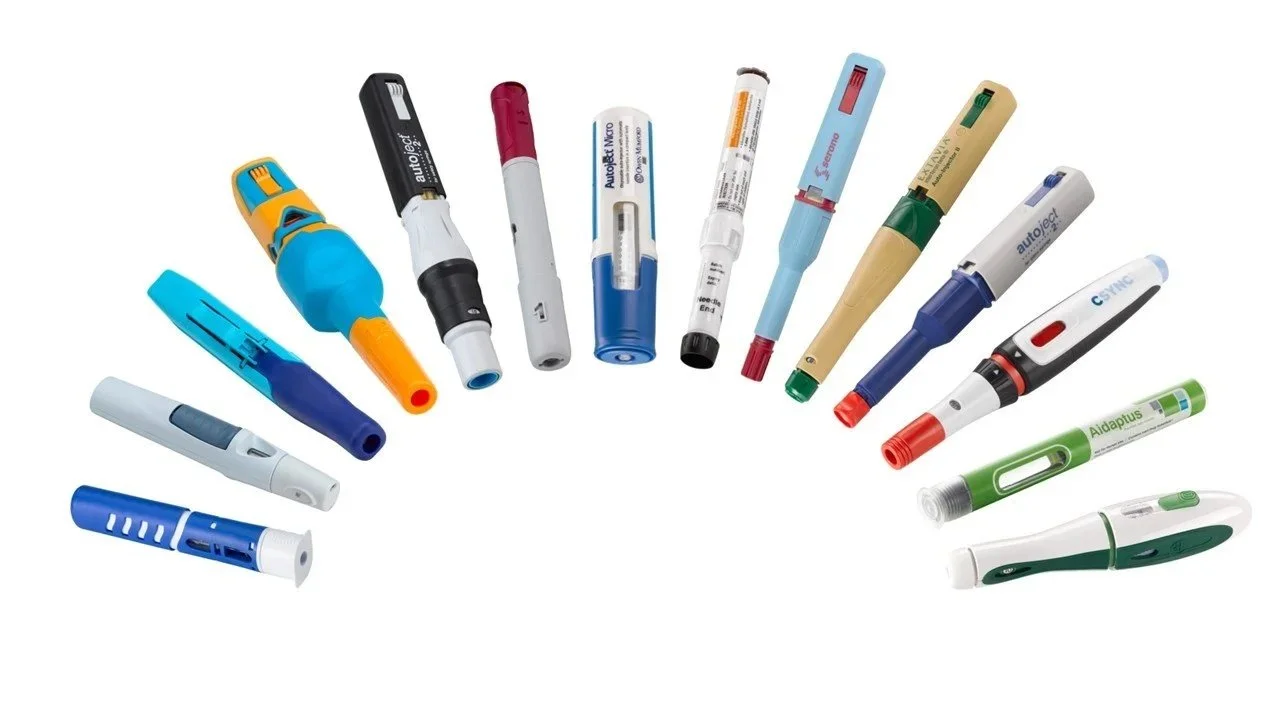
Our Services
Expert consulting services tailored to your business needs, providing strategic solutions and actionable insights. Enhance performance and drive CMC success with our proven professional.
Guiding and working with your organization to
- Resolve issues related to CMC
- CMC Development work either at your organization or at CDMO
- Serving interim role as CMC Head
About Us
BioPharm CMC Advisers, LLC brings decades of experience in developing small and large molecules from late discovery phase to product approval (BLA/NDA). The expertise gained from working on variety of modalities – monoclonal antibodies, bispecifics, ADC, oligonucleotides, mRNA, DNA, small molecules and practical knowledge having been involved with >100 IND/IMPD filing, multiple regulatory interactions/meeting and addressing issues pre-emptively allowing start-up and mid-size companies to take products into clinic and beyond.
Recently released complete response letter (CRL) by FDA suggest that many biotech companies fail to pay attention to the CMC resulting in clinical hold, delays, or even lack of approval. The data suggests that 74% of these CRL had CMC issues, compared to only 35% related to clinical or safety issues. Hence, a well thought, proactive CMC plan often saves both time and dollars. BioPharm CMC Advisers can be your support for your CMC success!
Why Should you work with us?
We bring practical experience and a track record of success …
Sandeep Nema is an experienced CMC professional who has worked at start-ups (SalioGen, Tessera Therapeutics), mid size company (Mallinckrodt) and large pharma (Pfizer / Pharmacia). He has led teams and guided them from discovery up to Phase 3 and registration. At Pfizer, Sandeep was responsible for establishing Protein Pharmaceutical R&D group that included Microbiology and Stability functions. During his tenure at Pfizer he was a part of Pfizer Comparability Expert Working Group, Impurity Council and clinical trial material labeling and distribution to the clinic.
Sandeep received his PhD in 1992 and since then has been involved with the development of small molecule, vaccines, gene therapy (mRNA, DNA) and protein drugs via Parenteral delivery. He has been lead formulator for four launched products.
Sandeep is a Certified Regulatory Affairs Professional. He is active in AAPS and PDA where he has served as Chairman, Arden House Conference on “Parenteral Products: Integrating Science, Innovation and Patient Needs”; Co-Chair, AAPS/FDA/USP workshop on ‘Future Direction in Aseptic Processing’; and past Chair of Sterile Product Focus group. He teaches at couple of Universities. Sandeep recently co-edited ‘Pharmaceutical Dosage Forms: Parenteral Medications”. In addition, he has served as Steering Committee Member for the Handbook of Pharmaceutical Excipients.
LinkedIn Profile: www.linkedin.com/in/sandeep-nema-5412942
Technical Areas of Service
Biologics (mAb, multi-specifics, proteins), vaccines, mRNA, DNA, and Small molecule
Process Development
Formulation Development
Analytical release and characterization methods
Dosage forms (solution, lyophilized, LNP, suspension)
Combination Products
Prefilled syringes, Cartridges, Autoinjectors
Cell line Development
Stability studies (ICH, in-use, photo, etc)
Specification setting
Freeze-drying
Process scale-up and Tech-transfer
Pharmacy Manual (Dosage and Administration)
Regulatory documents/meetings (IND, IMPD and Briefing book)
Contact us
Interested in working together? Fill out some info and we will be in touch shortly. We can’t wait to hear from you!
Website: www.BioPharmCMCAdvisers.com
E-mail: biopharmcmcadvisers@gmail.com
Phone: 314-239-5330